What do the vaccine efficacy rates indicate for our everyday lives and what do we expect from the vaccination?
COVID-19 Interactive Map: Vaccine tracker across Greece
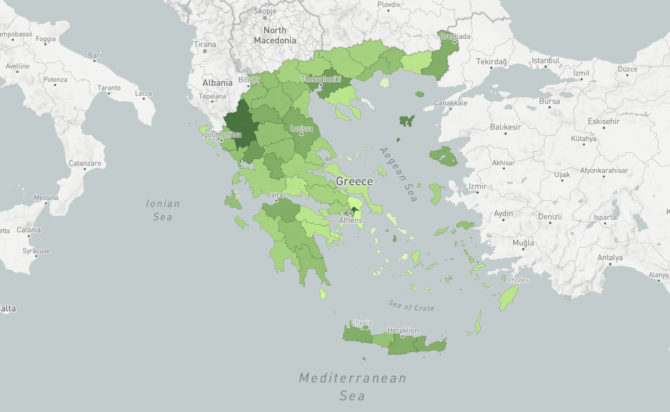
Real-time updates on vaccination per Greece’s Regional Unit
The spread of the disease in Greece and worldwide
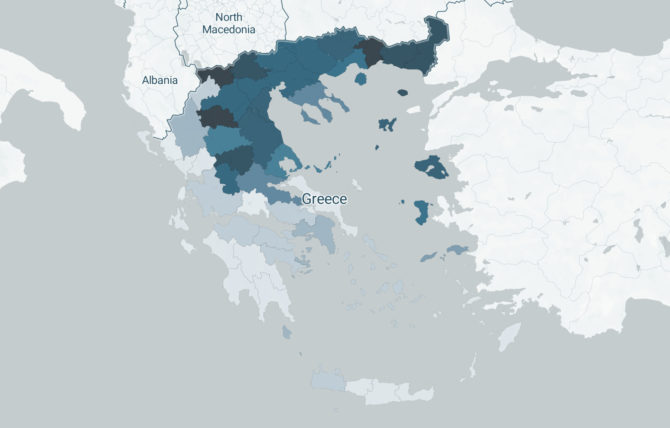
Constantly updated maps and charts showing the spread of the pandemic in our country and across the globe
How the application monitoring the spread of the disease was created
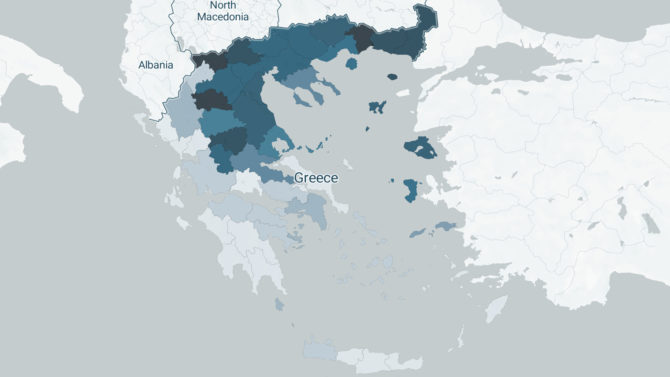
Work stages, sources/processing of data, tools and limitations regarding the development of iMEdD Lab’s web application tracking the pandemic in Greece and around the world.
Pandemic Death Toll Seems to Be Higher Than Reported
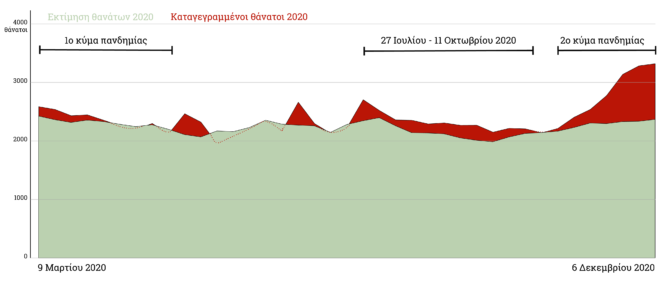
Increase in excess mortality in Greece, where a mere 43% of excess deaths were confirmed to be associated with COVID-19
As the time for the vaccination against COVID-19 has come, it is an opportunity to discuss what we expect from the vaccination and to clarify the meaning of the magic word “efficacy” of the vaccine, which was announced to be, on a case by case basis, as follows: 95% for the Pfizer vaccine1, 94.1% for the Moderna vaccine2 and 70% for the Astra Zeneca vaccine3. What do these numbers mean for our everyday lives?
The “efficacy” of a vaccine is the degree to which the vaccine protects against the disease and is usually expressed as a percentage: how many fewer infections we have in a group of people, who were actually vaccinated during clinical trials, compared to a “control group” who was given a placebo (dummy treatment) and therefore was not vaccinated.
For example, “95% efficacy” means that the group which got the vaccine developed 95% fewer COVID-19 cases than the one which did not get the vaccine. In the case of the Pfizer clinical trial, the number of people who received the vaccine was almost the same as the number of those who received a placebo –21,720 vs. 21,7281. In the latter, the “control group”, 162 cases of COVID-19 infection occurred at least seven days after the 2nd dose of the vaccine (Moderna and Astra Zeneca counted after at least 14 days). Thus, one would expect that, if the vaccine behaved like a placebo (that is, if practically it provided no protection), we would have about 162 patients, while, in the end, only 8 patients were recorded in the study –viz, in 95% (154/162) of the cases of patients we would expect, the vaccine offered protection and we avoided them.
Therefore, 95% of those who would get sick now will not get sick thanks to the protection by the vaccine. We must point out, however, that some people, even after being vaccinated, will get sick unless we can get a vaccine that is 100% effective.
It is also worth noting that these percentages apply to all study participants, but one can also measure the efficacy in subgroups, such as age groups.
What do we expect from now on? This 95% (or any other percentage) is usually not realized precisely in practice, that is in the general population: in English there are two different words that are used to express the notion of “efficiency”: “efficacy” for what happened in the clinical trial and “effectiveness” for the general population. Therefore, we anticipate that the “effectiveness” will be lower than the “efficacy”. The reason is that in a clinical trial the conditions are ideal: the participants are generally in better health, the nurses who administer the vaccine are more specialized, the storage of the vaccine is better, etc, compared to the everyday conditions.
Nevertheless, the efficacy demonstrated by the vaccines in the trials is very high. Let us not forget that the effectiveness of some of the flu vaccines is measured at 60%8. Also, if one reads the study protocols4,5,6 (the clinical protocols describe in striking detail what exactly will be done in a trial before its launch), the target for the vaccine to be considered successful was to demonstrate efficacy higher than 50%, according to the FDA guidelines7. Simply put, even if a vaccine demonstrated an efficacy of 50%, it could still be approved. All three companies designed their trial in this way4,5,6.
Here we should stress that in such clinical trials there are a number of safeguards, so that the final result can be attributed to the vaccine alone. For example, participants must meet specific criteria; enter the vaccination group or the “control group” at random and without knowing which group they belong to, so that we have balanced characteristics in both groups. Also, the number of participants has been determined in advance, so that we have enough information to minimize the possibility of seeing variations that are coincidental. And, of course, the participants are observed frequently for side effects.
A significant decrease in the possibility of contracting the disease, practically reduces the rate of transmission –therefore the famous R_t: the pandemic develops more slowly and –why not?– it fades away. To achieve this, of course, it is important to vaccinate a significant proportion of the population in a relatively short amount of time, as it is not yet clear how long is the immunity offered by the existing vaccines. The study over this very characteristic of them continues: that is, the participants continue to be monitored, in order for us to gain knowledge about the duration of immunity. In the forthcoming months we will have much better information about this as well.
1 Fernando P. Polack, M.D., Stephen J. Thomas, M.D., Nicholas Kitchin, M.D., et al., Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, The New England Journal of Medicine, December 31, 2020, https://www.nejm.org/doi/full/10.1056/NEJMoa2034577
2 Lindsey R. Baden, M.D., Hana M. El Sahly, M.D., Brandon Essink, M.D., et al., Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, February 4, 2021, https://www.nejm.org/doi/full/10.1056/NEJMoa2035389
3 Voysey M., Clemens A. C., Madhi S. A., et al., Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine, available at SSRN.
4 Pfizer’s protocol
5 Moderna’s protocol
6 Astra Zeneca’s protocol
7 FDA, Development and Licensure of Vaccines to Prevent COVID-19, June 2020, available fda.gov.
8 Rondy M., El Omeiri N., Thompson MG., Levêque A., Moren A., Sullivan SG., Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies, J Infect, November 2017, 75(5): 381-394, https://pubmed.ncbi.nlm.nih.gov/28935236/
Translation: Εvita Lykou